


 |
 |
 |
α-Linalool from Coriander Root Inhibits the Proliferation and Invasion of a Human Gastric Cancer Cell Line
Liping Xie1, Zhen’an Wu1, Yuna Liu1, Jiajian Tang2, Chen Lu3, Hongmei Wang2*
1Beijing Hospital of Integrated Traditional Chinese and Western Medicine, Beijing 100039, China. 2School of Medicine, Southeast University, Nanjing, 210009, China. 3Pukou Branch of Jiangsu People’s Hospital, Nanjing Pukou District Central Hospital, Nanjing, 210000, China.
Abstract
The goal of this study was to investigate the impact of coriander root extract on the migration and proliferation of the BGC-823 gastric cancer cell line, as well as to establish a theoretical and experimental foundation for the prevention and treatment of gastric cancer using coriander root. Clonal formation of BGC-823 cells was significantly reduced and cell proliferation was inhibited by coriander root extract; The invasion number of gastric cancer cells was reduced, the scratch healing ability was significantly reduced, and the cell migration ability was inhibited. The results showed that coriander root extract could inhibit the EMT process. After the action of coriander root extract, β-catenin and TGF-β/SMAD pathway genes like P-GSK-3β, TGF-β, and P-SMAD2/3 were decreased; The increased expression of SMAD2/3 and Gsk-3β suggested that coriander root extract may be mediated by β-Catenin and TGF-β/SMAD pathway regulated the proliferation and migration of BGC-823 cell of gastric cancer. Further analysis found that α- The human gastric cancer cell line BGC-823's growth and invasion were reduced by linalool. Coriander root extract, especially α-Linalool, which can be mediated by β-Catenin and TGF-β/SMAD pathway, inhibited the proliferation and migration of gastric cancer BGC-823 cells.
Keywords: Gastric cancer, Coriander root, α-Linalool, Proliferation, Migration
Gastric cancer refers to a malignant tumor derived from the epithelial cells of the human gastric mucosa. It is a common malignant tumor in the world at present. According to epidemiological data, stomach cancer incidence is fourth worldwide and the fatality rate is third, only second to lung and liver cancer.[1] Based on the World Health Organization (WHO) data in 2020, the prevealence and death rate of gastric cancer in China ranked first globally, accounting for 44.0% and 48.6% of the global incidence and mortality, respectively.[2] In 2021, there were about 1.05 million new cases of gastric cancer in the world.[3] As a high-incidence area of gastric cancer, China had 380000 new cases of gastric cancer in 2022,[4, 5] and its incidence and mortality ranked among the top three of all malignant tumors. Every year, there are about 679000 new gastric cancer patients in China, including about 498000 deaths as a result of gastric cancer. The incidence of gastric cancer in China is increasing yearly, and the 5-year survival rate is only 11.6%.[6, 7] The situation is very serious, and the prognosis is poor, which poses a serious threat to patient’s health and life safety and brings a heavy economic burden to the country.
At present, surgical resection, radiotherapy, and chemotherapy are commonly used to treat gastric cancer. Most of China's existing anticancer chemotherapy drugs, such as fluorouracil and doxorubicin, are not very selective. While They effectively inhibit and kill tumor cells, they also have certain inhibitory and killing effects on normal human tissue cells, especially hair follicles and human bone marrow cells with vigorous growth and proliferation.[8, 9] Chinese herbal medicine, especially Chinese herbal medicine with the same source of medicine and food, has the advantages of less systemic toxicity and side effects and is easy for patients to accept. Modern medicated diet, diet therapy, and health-keeping theory, which are further inherited and developed from traditional medicine and food and medical theory, have become the three health hotspots of general concern. Whether this theory can be applied to cancer prevention and treatment also provides a new direction for cancer-related research.
In 2022, the National Comprehensive Cancer Network of the United States issued the latest guidelines, proposing a treatment strategy with multidisciplinary participation and coexistence of multiple treatment methods, including surgery, perioperative chemotherapy, radiotherapy, targeted, and immunotherapy. Still, there are also some problems such as postoperative recurrence and metastasis, severe side effects, and reduced drug sensitivity. Due to their anti-cancer qualities, plant-based medications have recently generated a lot of interest and have shifted steadily into the centre of research. Previous research has demonstrated that the majority of medicinal and aromatic plants contain beneficial chemicals with distinctive qualities. Several widely used medications and substances, such as artemisinin, schisandrin C, paclitaxel, vincristine, and vinblastine, have been isolated from medicinal plants and used to treat a variety of ailments.[10-13] Under the guidance of the theory of traditional Chinese medicine, research in recent years has also found that some classic and famous prescriptions, clinical experience prescriptions, or chemical components isolated from Chinese herbal medicine have proved to have significant advantages in preventing and treating gastric cancer. Studies have confirmed that curcumin, a natural polyphenol product extracted from plants, has clear anti-gastric cancer effects.[14] The prevention and management of stomach cancer by traditional Chinese medicine is the feature and advantage of treating gastric cancer in China. In recent years, Chinese medicine has achieved good results in treating gastric cancer, showing a good prospect. Its multi-mechanism and multi-level therapeutic effect have also been widely considered and valued. Many clinical literatures proven that Chinese medicine have an active role in preventing and treating gastric cancer, especially in improving clinical symptoms, preventing the further development of precancerous lesions, preventing recurrence and metastasis after surgery, and improving chemotherapy's effectiveness and lowering its toxicity. We are therefore eager for a gentle and successful course of Chinese medicine treatment.
Coriandrum sativum, also known as coriander, is among the most commonly employed spices in cooking globally. Coriander is an annual plant with green lanceolate leaves, obvious main roots, white or pink umbels, 20-70cm high, spherical dry seeds, widely planted in Africa, Asia, and Europe. Numerous research have revealed a wide range of biological activity for coriander extract and its bioactive compounds, including antioxidant, anti-cancer, neuroprotective, anticonvulsant, analgesic, and anti-inflammatory properties.[15-17] The therapeutic value of coriander and its combination with people's daily life make coriander a unique functional food. These medicinal values of coriander are mainly mediated by the strong antioxidant activity of coriander extract and its main compound-linalool.[18-20] When Tang et al. studied the anti-proliferation activities of extracts from different parts of coriander root, leaf, and stem against breast cancer cells MCF-7, they found that the ethyl acetate extract of coriander root consist of the maximum content of phenols, the strongest antioxidant and anti-proliferation activities. They inhibited the migration of breast cancer cells in a certain dose-dependent way. Moreover, the study also found that coriander leaf extract can inhibit the proliferation and migration of prostate cancer cells PC-3 and LNCaP.[17] Eid AM et al. prepared coriander oil into nanogel to obtain nanoemulsion with a polymer dispersion coefficient of 0.188 and particle size of 165.72nm, which has obvious anti-cancer effect on human breast cancer cell MCF-7, liver cancer cell Hep3B, and cervical cancer cell HeLa. Huang et al. studied the effect of coriander on the human hepatoma cell line (HepG2) and mouse melanoma cell line (B16F10). They found that coriander extract can effectively inhibit the migration and invasion of cancer cells and improve the prognosis of cancer patients. Amrita Devi Khwairakpam et al. found that Vietnamese coriander has anti-proliferation, keeping-survival, and anti-metastasis activities, and induces cell cycle arrest in the G2 phase.[21] At the same time, it can inhibit the AKT-mTOR signaling pathway, and downregulate the expression of cyclin D1, cyclooxygenase-2 (COX2), survivin, matrix metalloproteinase-9 (MMP-9), vascular endothelial growth factor-a (VEGF-A) and other key proteins involved in tumorigenesis. It has been studied that Vietnamese coriander can inhibit human oral cancer progression by dose-dependent down-regulation of the AKT-mTOR signaling pathway.[21] Related studies also found that coriander plays an anti-cancer role in many human cancers, such as colon, breast, and cervical cancer.[22, 23] However, the function of coriander in the progression of gastric cancer and its related mechanisms has not been reported yet. In order to provide a theoretical and experimental foundation for the prevention and treatment of gastric cancer by coriander root, the effects of coriander root extract on the proliferation and migration of gastric cancer cells were examined in this study using molecular biology, immunology, and other techniques.
Materials and Methods
The Etiology Laboratory of Jiangsu University School of Medicine donated gastric cancer cell BGC-823. Fetal bovine serum and DMEM culture medium were purchased from Gibco Company; Trypsin was purchased from Sigma; GAPDH antibody was purchased from Abcam Company; Protein marker was obtained from Shanghai Biochemical Reagent Co., Ltd., and Ab such as MMP9, MMP2, PCNA were purchased from Wanke Biological Co., Ltd.
Rinse and dry the coriander root, weigh it, soak it in the proper amount of double distilled water for 35 minutes, add a total amount of 3l double distilled water, and decoct it with the conventional Chinese medicine decocting method. When decocting to 200ml liquid, stop heating, calculate the concentration of coriander decoction (the final drug concentration is 14.315mg/ml), and use it at - 20 ℃ for standby.
Human gastric cancer cell BGC-823 was cultured in an incubator with 37°C and 5%CO2, When the cell fusion degree reached 80-90%, it was digested, resuspended into a single cell, and then counted; 4×104/well cells were inoculated in a 12 well plate and cultured overnight in a 37°C, 5% CO2 incubator. Cells were incubated with coriander root extract with a final drug concentration of 0.01, 0.02, 0.04, 0.08, and 0.16mg/ml, respectively. After 24, 48, and 72 hours of treatment, count the cells and draw a curve chart with the coriander root extract concentration cell number.
The drug treatment group received therapy with DMEM culture medium and various concentrations of coriander decoction, while the control group received treatment with DMEM culture medium only, the concentrations were 0.01, 0.02, 0.04, 0.08, 0.16 mg/ml, respectively, and their OD values were measured after 48 hours of treatment at the same time. Another drug treatment group was managed with DMEM culture medium with coriander decoction of the same concentration (0.02mg/ml), and their OD values were measured at 12,24,36,48,72 h, respectively.
BGC-823 cells in the logarithmic growth phase were inoculated into 96 well plates, with 1000 cells per well, and 3 double wells were set in each group; After the cells adhered to the wall the next day, the blank control group was not treated with drugs. The coriander decoction-treated experimental group was split into two groups. The first group was treated with different concentrations of coriander decoction simultaneously. The concentration of coriander decoction was 0.01, 0.02, 0.04, 0.08, and 0.16 mg/ml, and its OD value was measured after 48 hours of treatment at the same time; The second group had the same drug concentration for the different action times. The concentration of coriander decoction was 0.02mg/ml, and its OD value was measured at 12,24,36,48,72 hours respectively.
Human gastric cancer BGC-823 cells in the logarithmic phase were inoculated into 6-well plates with 3.0×105cells per well; The next day was treated according to the experimental groups, which were divided into the blank control group and the drug treatment group. The drug concentration of the drug treatment group was 0.02mg/ml. After 48 hours of drug action, the cells were digested and resuspended into a single-cell suspension. Following a count, 1000 cells per hole were injected into a fresh 6-hole plate. The culture media was replaced every three to four days while the cells were cultivated in the cell incubator for 10 to 12 days. Discard the culture medium. After PBS washing, cells were used paraformaldehyde to fix at room temperature for thirty mins. After 30min, discard the paraformaldehyde. PBS washing, crystal violet staining at room temp for 15 mins, PBS washing, drying at room temperature, and taking photos to analyze the results to calculate the clone formation rate.
The logarithmic growth human gastric cancer BGC-823 cells were inoculated into a 6-well plate with 3.0×105 cells per well. The next day, the cells were treated according to the experimental groups. After 48 hours of digestion, the serum-free cell suspension was prepared. Each transwell cell was inoculated with 1x105 cells in the upper chamber and 600 μL with serum culture medium in the lower chamber, culturing in 37°C, 5% CO2 incubator for 24h. PBS was washed and fixed with 4% paraformaldehyde for 30min. PBS was washed and dyed with crystal violet for 15 minutes. After PBS was washed, cells in the upper room were wiped off with a cotton swab. After drying at room temp, they were examined under a microscope, and 3–4 randomly chosen visual fields were photographed.
The logarithmic growth human gastric cancer BGC-823 cells were inoculated into a six-hole plate with 3.0×105 cells per hole. The next day, they were treated according to the experimental groups. After 48h, the cells were lysed with protein lysate containing protease inhibitor for 30min, and centrifuged at 13000rpm for 30min at 4 ℃; The supernatant was mixed with 5xloading Buffer at 4:1, placed in a 100 ℃ water bath to boil them for 10min, and then placed them in a - 20 refrigerator for standby.
10% SDS-PAGE gel was prepared, and was loaded with the protein sample; The Protein was transferred to PVDF membrane under 300 mA constant current for 90 minutess; After the completion of membrane conversion, it was placed in 5% skimmed milk powder at room temperature and sealed for 2h. It was incubated The first antibody at 4°C, overnight. Image Pro Plus software was used for quantitative analysis.
The experimental results were statistically examined with GraphPad Prism 5.0 software, and the data were all expressed in mean ± standard deviation (X ± SD). Student's t-test or one-way ANOVA was used to compare the experimental groups, and ns had no statistical significance, * p<0.05, * * p<0.01, * * * p<0.001.
At three separate drug action durations of 24, 48, and 72 h, the cell count data demonstrated that, in comparison to the control group, the drug suppressed the proliferation of gastric cancer BGC-823 cells in a concentration-dependent manner. According to the statistical analysis, the difference between the cell count values of 0.02mg/ml and 0.01mg/ml drug concentration at the time of action of each drug was statistically significant (p<0.05), and the difference was most significant at 48h (p<0.005). Therefore, the subsequent experiment (Figure 1) was conducted after the drug concentration of 0.02mg/ml was used for 48h.
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
Figure 1. Statistical chart of cell count results. (a) Effects of coriander extract of different concentrations on the proliferation of gastric cancer BGC-823 cells after 24 hours. (b) Effects of coriander extract of different concentrations on the proliferation of gastric cancer BGC-823 cells 48 hours later. (c) Effects of coriander extract of different concentrations on the proliferation of gastric cancer BGC-823 cells 72 hours later. * p<0.05, * * p<0.01, * * * p<0.001. |
To analyze the effect of coriander root extract on the proliferation of gastric cancer cells, CCK8 results showed that coriander root extract hinder the proliferation of gastric cancer BGC-823 Compared to the control group, cells were affected by time and concentration. The drug concentration of 0.02mg/ml for 48h could hinder the proliferation of gastric cancer BGC-823 cells by half. In addition, BGC-823 cells have moderate vitality, which is suitable for the subsequent study of related phenomenon experiments and functional experiments. Therefore, this action condition is taken as the basic condition for the subsequent study (Figures 2a and 2b); The results of plate cloning showed that after 2 weeks of cell culture, the number of BGC-823 cell clones formed by 0.02mg/ml coriander root extract for 48 hours significantly decreased. The volume became smaller (Figure 2c), (*p<0.05).
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
Figure 2. CCK-8 test detects the effect of coriander root extract on the proliferation of BGC-823. (a) CCK-8 test was used to detect the effect of coriander extract of different concentrations on the proliferation of gastric cancer BGC-823 cells after 48 hours of treatment. (b) CCK-8 test was used to detect the effect of coriander extract of the same concentration on the proliferation of gastric cancer BGC-823 cells 48 hours later. (c) The effect of 0.02 mg/ml coriander extract on the cloning ability of gastric cancer BGC-823 cells 48 hours later. |
We confirmed the associated genes that influence cell proliferation in order to further investigate the molecular mechanism by which coriander root extract inhibits the growth of gastric cancer cells. Western blot results showed that PCNA and C-MYC expression levels significantly reduced after the 0.02mg/ml coriander root extract acted for 48 hours compared with the control group. It shows that coriander root can inhibit the proliferation of gastric cancer BGC-823 cells (Figure 3a), (* p<0.05, * * p<0.01).
Furthermore, the β -Catenin signaling pathway-related genes, β
-Catenin signaling pathway-related genes, β -Catenin, GSK-3β
-Catenin, GSK-3β , and P-GSK-3β
, and P-GSK-3β , were detected. The results showed that β
, were detected. The results showed that β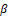 -Catenin and P-GSK-3 declined, and the expression level of GSK-3β was increased compared with the control group. In summary, coriander root extract regulates the proliferation of gastric cancer cells by the β
-Catenin and P-GSK-3 declined, and the expression level of GSK-3β was increased compared with the control group. In summary, coriander root extract regulates the proliferation of gastric cancer cells by the β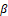 -Catenin signaling pathway (Figure 3b), (* p<0.05, * * p<0.01).
-Catenin signaling pathway (Figure 3b), (* p<0.05, * * p<0.01).
|
|
|
a) |
|
|
|
b) |
|
Figure 3. Effect of coriander root extract on proliferation-related pathway proteins of gastric cancer cell BGC-823. (a) The expression changes of proliferation-related protein, PCNA, C-myc. (b) Expression changes of β-Catenin pathway-related protein. |
Transwell migration experiment results showed that the number of cells passing through the membrane per unit area decreased after the treatment of coriander root extract compared with the control group, indicating that coriander root extract can inhibit the invasive ability of human gastric cancer BGC-823 cells (Figure 4a); The scratch test finidngs proves that in comparison to the control group, the ability of cell scratch healing was significantly reduced after the treatment of coriander root extract, indicating that coriander root extract could inhibit the incursion of gastric cancer BGC-823 cells (Figure 4b).
|
|
|
a) |
|
|
|
b) |
|
Figure 4. The effect of coriander root extract on cell migration ability. (a) The effect of 0.02mg/ml coriander root extract on cell migration ability was detected by the transwell test. (b) The scratch healing test was used to detect the effect of 0.02mg/ml coriander root extract on the scratch healing ability of cells |
Furthermore, Classical genes associated with invasion were examined as follows. WB results showed that in comparison to the control group, the expression of MMP2 and MMP9 decreased, the expression of epithelial marker E-cadherin increased, and the expression of interstitial cell-related markers N-cadherin, Vimentin, and Snail reduced, representing that coriander root can hinder the EMT process of stomach cancer BGC-823 cells and inhibit the invasion of gastric cancer cells (Figures 5a and 5b); The expression level of TGF-β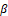 and P-SMAD2/3 was reduced, and the expression level of SMAD2/3 considerably surged. In summary, coriander root extract may regulate the invasive ability of gastric cancer BGC-823 cells via the TGF- β
and P-SMAD2/3 was reduced, and the expression level of SMAD2/3 considerably surged. In summary, coriander root extract may regulate the invasive ability of gastric cancer BGC-823 cells via the TGF- β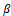 /SMAD signaling pathway (Figure 5c), (* p<0.05, * * p<0.01, * * * p<0.001).
/SMAD signaling pathway (Figure 5c), (* p<0.05, * * p<0.01, * * * p<0.001).
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
Figure 5. Effects of coriander root extract on gastric cancer cell invasion and expression of related pathway proteins; (a) Changes of protein MMP2 and MMP9; (b) Changes of EMT related proteins; (c) TGF-β/The changes of SMAD pathway protein. |
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
|
|
d) |
|
|
|
e) |
|
Figure 6. Effects of α-Linalool on gastric cancer cell proliferation and invasion; (a) CCK-8 test was used to detect the effect of α-Linalool of different concentrations on the proliferation of gastric cancer BGC-823 cells after 48 hours of treatment. (b) CCK-8 test was used to detect the effect of α |
Cancer is a disease characterized by uncontrolled growth and dysfunction of tumor cells, which is partly due to the proliferation of tumor cells with a metabolic disorder. Because tumor cells have a variety of characteristics, such as vigorous metabolism, division and proliferation, and strong migration ability, they tend to progress rapidly once they occur.[24, 25] An essential factor in the development of cancer is the proliferation, invasion, and metastasis of tumour cells in the middle and late stages of the disease, development, and malignant transformation of human tumors, especially the metastasis of advanced cancer, which has gradually become the leading cause of morbidity and mortality of cancer patients.[26, 27] Therefore, the main purpose of most anti-cancer treatments is to hinders the proliferation and invasion of tumor cells.
At present, the treatment of cancer is mainly surgery, radiotherapy, and chemotherapy. The systemic toxicity and side effects of chemotherapy drugs are the main problems commonly existing in the radiotherapy and chemotherapy treatment of malignant tumors, seriously affecting patients' quality of life.[9] Traditional Chinese medicine has the advantages of relatively small systemic toxic and side effects, direct action site, significant efficacy, etc. These advantages make traditional Chinese medicine popularized and applied in treating malignant tumors. In recent years, with the extensive application and further in-depth research of aromatic drugs, their aromatic chemical components have played an important role in many aspects such as antibacterial, antioxidant, anti-tumor, anti-virus, and promoting the absorption of drugs by the body.[28-33] The coriander's root has various aromatic chemical components, rich furfural, unsaturated fatty acids, and other helpful chemical components for the human body.[34-36]
In this investigation, we looked at how coriander root extract affected the growth and migration of a stomach cancer cell line. BGC-823 in vitro.CCK-8 and plate cloning experiments showed that coriander root extract effectively inhibited the proliferation of stomach cancer cell BGC-823; Western blot showed that the expression of PCNA (proliferating cell nuclear antigen) and C-myc protein associated to cell proliferation was downregulated. β-Catenin is a subunit of the cadherin complex. Its main role is to mediate the adhesion between cells and participate in the synthesis and expression of genes. In the Axin complex, Gsk-3βCan effectively make β-Catenin phosphorylation, where agglomeration of catenin will effectively activate the Wnt/β-Catenin pathway, which can induce tumorigenesis. testing the core protein of the β-catenin signaling pathway found that the expression of related proteins changed after the action of coriander root extract. Therefore, it is speculated that coriander root may be throughβ-Catenin signaling pathway that inhibits the proliferation of gastric cancer cells.
EMT (epithelial-mesenchymal transformation) is the ability of epithelial cells to change into mesenchymal cells in morphology and obtain migration. EMT is the basic process of embryonic development and the basis of normal development, wound healing, and malignant tumor occurrence.[37-39] It can enhance the invasiveness of cells, improve their stem cell characteristics, and inhibit the occurrence of tumor cell apoptosis, which may eventually lead to tumor deterioration. Western blotting proves that coriander root extract considerably inhibited the EMT transformation of BGC-823 cells.[39] Studies have shown that tumor-related cells can secrete many matrix metalloproteinases (MMPs), damaging the cell basement membrane and promoting tumor tissue invasion. Transwell chamber migration and scratch experiments show that coriander root extract hinders the incursion of stomach cancer cells after the effect of coriander root extract. Western blot experiments more to verify that the expression levels of MMP2 and MMP9 are reduced. At the same time, by detecting TGF-β/Core protein TGF in SMAD signaling pathway-β、the expression of SMAD2/3 and P-SMAD2/3 showed that TGF-β、the decrease of P-SMAD expression and the increase of SMAD expression further verified that coriander root extract might pass TGF-β/The SMAD signaling pathway regulates the invasiveness of gastric cancer cells.
A natural terpene alcohol called linalool is present in many fruits, spices, and herbs. Antibacterial, anti-inflammatory, and antioxidant effects of linalool have been noted. In addition, linalool has anti-cancer potential for prostate, colon, leukemia, and cervical cancer.[40-42] Linalool's apoptotic action, oxidative stress induction, cell cycle arrest, and immunological modulation may all contribute to its anticancer effects.[43] In DU145 and PC-3 prostate cancer cells, linalool can induce cell cycle arrest and external death receptor-dependent apoptosis pathway. Linalool was found to inhibit the phosphorylation of ERK1, JNK, and p38 proteins of the mitogen-activated protein kinase family and block the activation of nuclear factors κ B/p65 to protect HDFa cells from oxidative stress. Linalool also stimulates interferon γ
B/p65 to protect HDFa cells from oxidative stress. Linalool also stimulates interferon γ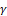 , Interleukin (IL) - 13, IL - 2, IL - 21, IL - 21R, IL - 4, IL - 6sR and tumor necrosis factor (TNF-α
, Interleukin (IL) - 13, IL - 2, IL - 21, IL - 21R, IL - 4, IL - 6sR and tumor necrosis factor (TNF-α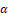 ) Secretion. P53 and cyclin-dependent kinase inhibitors were up-regulated in linalool-treated leukemia cells. moreover, caspase-3 and caspase-9 were activated in linalool-induced apoptosis and death of glioma cells.
) Secretion. P53 and cyclin-dependent kinase inhibitors were up-regulated in linalool-treated leukemia cells. moreover, caspase-3 and caspase-9 were activated in linalool-induced apoptosis and death of glioma cells.
This study was to investigate the antiproliferative effect of linalool on gastric cancer cells and its mechanism. The efficacy of this compound was evaluated and compared in vitro cell line models.[44-49] In conclusion, α-Linalool of coriander root extract may be mediated by β-catenin and TGF-β/SMAD signaling pathway inhibits the proliferation and migration of stomach cancer cell BGC-823. However, its specific mechanism in gastric cancer still needs further experimental research to verify. Coriander root may become a new idea for the clinical prevention and management of stomach cancer.
We would like to express our gratitude to the editors and the anonymous referees for their work in reading and evaluating our paper.
None.
This work was financially supported by the Zhishan Scholars Programs of Southeast University (2242021R41070).
The ethical guidelines and human welfare committees of Beijing Hospital of Integrated Traditional Chinese and Western Medicine requested and authorised all experimental procedures.
|
||||||||